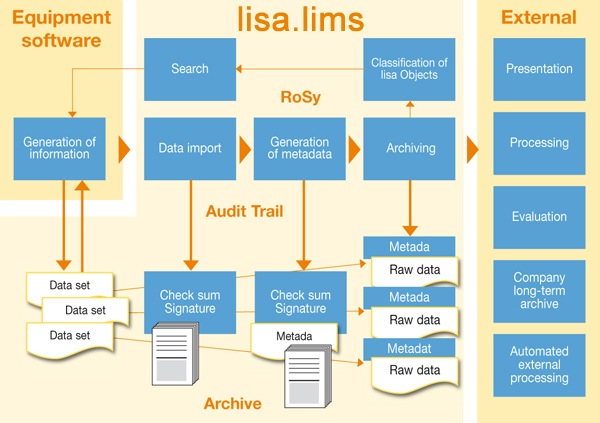
The lisa.lims module RoSy – systematic raw data archiving
RoSy is an extension of lisa.lims for archiving raw data effectively in the regulated environment. The key advantage of raw data archiving is the optimization of process operations and a considerable reduction in paper-based archiving work in the laboratory.
RoSy meets the stringent FDA 21 CFR 11 and is therefore qualified for use in laboratories with complex testing and archiving processes, particularly at pharmaceutical companies and their suppliers.

Fully automated data archiving with RoSy
RoSy fully automates archiving of key electronic data generated by sophisticated analytical instruments and subsystems.
The program module’s comprehensive approach is based on a user-specific archiving strategy, which takes into account all individual processes and requirements as well as the client’s wider IT environment. The system not only stores the files and reports, but, depending on the configuration you choose, also assigns them to the next processing steps and approval processes as required. This makes RoSy ideal for use across different locations, especially in a diverse IT environment.